Desulfurization of liquid fuels over activated carbons
- prativabehera768
- Apr 26, 2025
- 2 min read
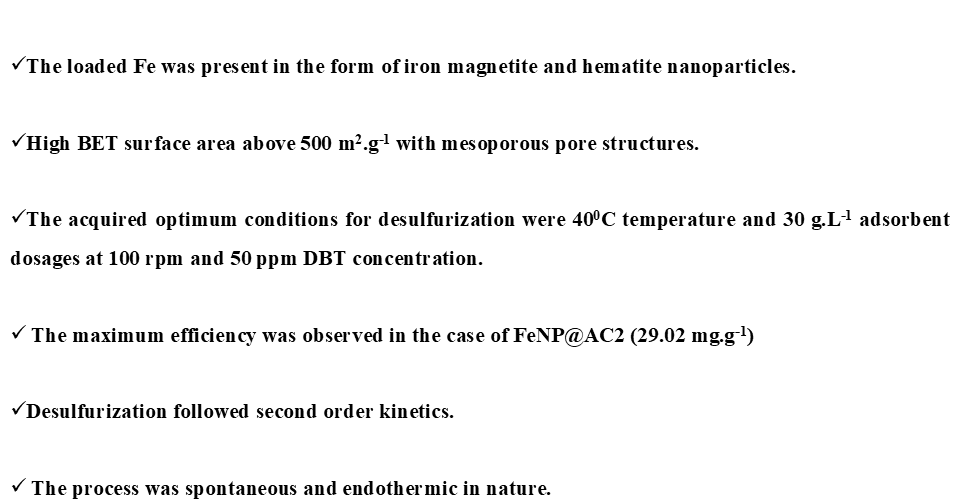
The combustion of fossil fuels in automobiles and several industries release toxic gases of oxides of sulfur and CO2 to the environment. These gases may cause environmental pollution, global warming and severe health issues. Fossil fuels comprise of various naturally occurring sulfur compounds and hydrocarbons. Indian government with environmental regulating bodies have introduced stringent norms to limit the sulfur standard. To meet the current norms, the conventional hydrodesulfurization process can be integrated with adsorptive desulfurization. In the present work, we have modified the commercially available activated carbon DARCO, to selectively target sulfur compounds in liquid fuels. Iron nanoparticles (FeNP) were selected as a modifying agent. These FeNPs were loaded on both, granular and powdered form of the AC and the efficiency of these prepared materials in the removal of sulfur compound i.e. Dibenzothiophene (DBT) was examined. The model oil was prepared by dissolving DBT in hexane. The characterization of the adsorbent was studied using standard methods to understand the surface physical and chemical nature. The powdered FeNP@AC was found to be magnetic in nature. Batch adsorption experiments were carried out using model oil to study the effects of adsorbent dosage, contact time, temperature and initial concentration of DBT. The optimum conditions for desulfurization were found to be at temperature of ~400C and dosage of 30 g.L-1. The adsorption capacity for the different adsorbents for the desulfurization of DBT followed the order as:
Granular AC (AC1) < Granular FeNP@AC (FeNP@AC1) < Powdered AC (AC2) < Powdered FeNP@AC (FeNP@AC2)
The Tempkin adsorption isotherm could well describe the adsorption mechanism of both the nascent activated carbon while Langmuir adsorption isotherm could define DBT adsorption at the FeNP@AC1. It is interesting to note that the FeNP@AC2 followed the Freundlich adsorption isotherm, which implies a heterogeneous adsorbent surface. The kinetic study revealed that the pseudo second order model could describe the surface binding mechanism of DBT. Multiple steps were involved in the rate limiting step, as indicated by the Weber-Moris plot. It was observed that the adsorption capacity of the prepared materials was increased with increasing initial concentration of DBT.








































































Comments