Introduction to Computational Chemistry, Part 1: Hartree Fock Theory
- prativabehera768
- Apr 30
- 1 min read
Hartree–Fock (HF) theory is a foundational approach in quantum chemistry for approximating the solution to the Schrödinger equation in systems with multiple electrons, such as atoms and molecules. It simplifies the complex issue of electron–electron interactions by assuming that each electron moves within an average field generated by all other electrons.
Key Concepts of Hartree–Fock Theory:
Mean-Field Approximation:
Each electron experiences the average effect of other electrons instead of instantaneous repulsion.
This results in a set of equations where electrons are treated independently.
Slater Determinant:
The wavefunction is expressed as a single Slater determinant to uphold the Pauli exclusion principle (antisymmetry of the wavefunction).
Self-Consistent Field (SCF) Method:
The HF equations are solved through an iterative process.
Guess orbitals → build Fock matrix → solve → obtain new orbitals → repeat until convergence.
Fock Operator:
An effective one-electron operator that includes kinetic energy, nuclear attraction, and average electron repulsion.
Limitations:
Ignores electron correlation beyond the mean-field approximation.
Often addressed by post-Hartree–Fock methods (e.g., MP2, CI, CCSD) or density functional theory (DFT).





















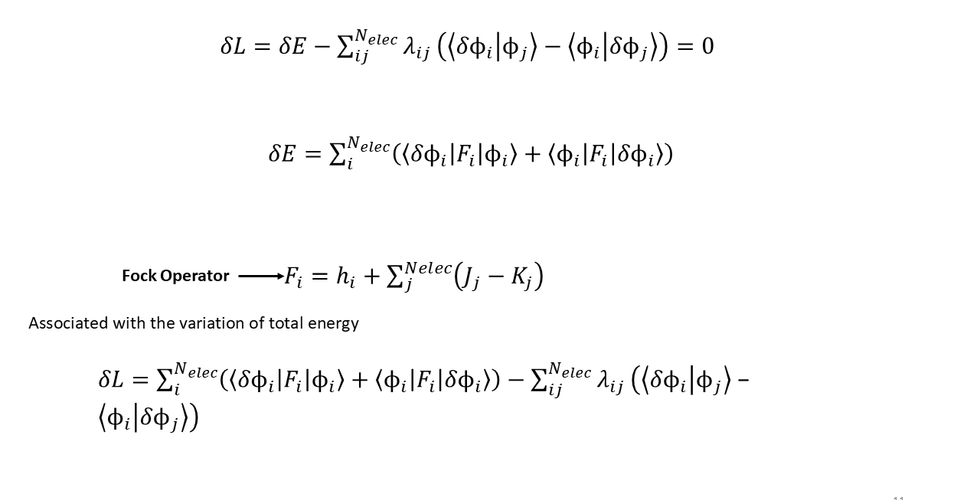









Comments